

Heisenbergs Principle does not pertain to the power fields created by the atomic nuclear structure, but rather to the position of the electrons within these power fields. No uncertainty is needed for that aspect of our world. Heavier isotopes react slower in enzyme-catalyzed reactions but they form stronger bonds. The structure of atoms is known to us in great detail down to the fact that isotopes are not totally equal to the major form of an element. Both spin-lattice and spin-spin relaxation mechanisms can contribute to the overall lifetime. The resulting line-shape changes yield information about the chemical environment of the Mn atoms. Excited-state lifetimes are subject to environmental (including chemical) influences. Spectral line width varies inversely with the excited-state lifetime according to Heisenbergs principle, AT X A H = hi 2n, where AT is the lifetime of the excited spin state, h is Planck s constant, and AH is the effective width of the absorption signal. And also, the femtosecond laser radiation allows the photoion to be photoselectively extracted from certain parts of a molecule. It must be emphasized again that the key difference lies in the fact that for photoion microscopy there is no need for a strong (ionizing) electric field that distorts and desorbs the molecules. The same factors restrict the spatial resolution of the field-ion microscopy.
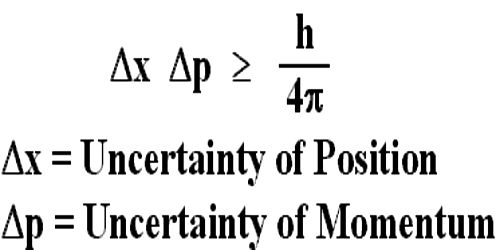
The resolution of photoion laser microscopy is limited by two fundamental factors the Heisenberg principle of uncertainty and the presence of the nonzero tangential component of the velocity of the ejected photoion (photoelectron). According to the Heisenberg principle, if the excited state has a lifetime of A/, then this results in a minimum uncertainty for the energy AE, such that AEAt > h/lit.
When an atom emits radiation, the frequency of the corresponding photon is linked to the energy of the levels between which the transition occurs. The Heisenberg principle tells us that the energy uncertainty of a given state is inversely proportional to the uncertainty in the time during which the corresponding energy level is occupied. Consider the double-slit patterns obtained for electrons and photons in Figure 12.24.Neuhauser D 1994 Circumventing the Heisenberg principle-a rigorous demonstration of filter-diagonalization on a LiCN model J. Let us explore what happens if we try to follow a particle. It is somewhat disquieting to think that you cannot predict exactly where an individual particle will go, or even follow it to its destination. Those who developed quantum mechanics devised equations that predicted the probability distribution in various circumstances. There is a certain probability of finding the particle at a given location, and the overall pattern is called a probability distribution. After compiling enough data, you get a distribution related to the particle’s wavelength and diffraction pattern. However, each particle goes to a definite place (as illustrated in Figure 12.23). The idea quickly emerged that, because of its wave character, a particle’s trajectory and destination cannot be precisely predicted for each particle individually. Both patterns are probability distributions in the sense that they are built up by individual particles traversing the apparatus, the paths of which are not individually predictable.Īfter de Broglie proposed the wave nature of matter, many physicists, including Schrödinger and Heisenberg, explored the consequences. Figure 12.24 Double-slit interference for electrons (a) and photons (b) is identical for equal wavelengths and equal slit separations.


 0 kommentar(er)
0 kommentar(er)
